
Task: Compose a Cell Profile Report (Introduction and Discussion) on Gonocytes, which originate from Germ Cells in the male testis. Essential guidelines are presented for composing each component, together with first references supplied.
I have also included an example cell profile report on several cell types. The assignment's marking rubric is also provided.
Comprehensive instructions are outlined below:
Cell type - Gonocytes (a concise presentation on gonocytes is included in the attached PDF).
This part must have around three pages, inclusive of illustrations.
Gonocytes are the primordial germ cells that serve as the precursors of gametes in the developing gonads. They are crucial for the establishment of the reproductive system and play a significant role in the formation of sperm and ova. These cells undergo a series of developmental stages, ultimately leading to meiosis and the generation of mature gametes, therefore ensuring the continuity of genetic information throughout generations.</text
What is the role of gonocytes? Does the function vary between the embryo and the adult?
3) What is the location of Gonocytes?
Select the location where Gonocytes are located and concentrate on it, while also indicating their presence in other areas of the embryo.
4) What procedures does it undergo throughout embryogenesis (and thereafter) to develop?
the ultimate, operational Gonocyte? Describe the development of gonocytes from fertilization forward. Process of germ cell development inside the testis
5) Which additional tissues must Gonocytes engage with to perform their functions? Consider not just proximal tissues but also significant distal interactions, such as hormones secreted by numerous glands. Signaling – FGF-2/Notch signaling in seminiferous epithelium; LH – facilitates apoptosis; FSH – induces ABH production by Sertoli cells.
6) Describe the components of cell-cell adhesion, proliferation, apoptosis, and/or migration.
Crucial for the maturation of this cellular type (e.g., Sertoli cells, Leydig cells; Apoptosis - sustains the ratio of Sertoli cells; SSC undergo proliferation.)
7) Present preliminary facts on your Discussion subject.Definition of cryptorchidism, prevalence percentage, and a brief discussion on its association with infertility, emphasizing the need for attention to male reproductive health.
Subject – Cryptorchidism
This part should include around three pages and concentrate on developmental aspects.
Biology of gonocytes, with photos.
Several essential elements that the conversation should encompass: (other points may be included).
The Gonocytes assignment report analyzes many features of Gonocytes, which serve as progenitors to spermatogonia. Prior to their differentiation into spermatogonial stem cells, these cells are present from week 7 of embryonic development to the newborn period. Male germ cells facilitate inheritance via the transmission of epigenetic and genetic information between generations. Spermatogonia are essential for male fertility, which relies on a substantial population of stem cells. The quality and functionality of a sperm cell are contingent upon its origin, namely the spermatogonial stem cell (SSC).
During the migratory developmental phases, germ cells are represented by gonocytes, which are transient and sequential. These phases occur from the suppression of gonad production in the genital area to their migration to the basement membrane of the seminiferous cords. The progression of Gonocytes is categorized into several phases: cellular proliferation, differentiation, migration, and apoptosis. Any irregularity throughout these phases may result in a fertility-related disorder.
Prior to establishing themselves in the gonads, Primordial Germ Cells (PGC) undergo many rounds of proliferation in the testes before differentiating into gonocytes. The proliferation of gonocytes persists post-colonization, accompanied by a simultaneous surge of apoptosis. Cyclin B1 is essential for gonocyte proliferation, which is necessary for normal spermatogenesis. Numerous studies indicate that a deficiency of cyclin B1 during postnatal development results in a reduction of germ cells owing to heightened apoptosis and mitotic arrest. Proliferation is suppressed in most gonocytes when spermatogonial stem cells are present 17 days post-conception, resuming mitosis after delivery. In this phase, as noted in the Gonocytes assignment section, proliferative post-natal and fetal gonocytes undergo apoptosis, whereas no other gonocytes perish. Transforming Growth Factor ? (TGF?) signaling is crucial in spermatogenesis, since it regulates several processes associated with germ cells, including death, differentiation, and proliferation.
Notably, DNA-damage-inducible-45-alpha (Gadd45?) and growth arrest are specifically expressed in germ cells. Investigations into the Gonocytes assignment case scenario have shown that, in conjunction with somatic cytochrome c (Cycs), Gadd45? expression enhances the differentiation of gonocytes. Any deactivation in the expression of these two genes may result in unregulated proliferation and susceptibility to malignancy. These genes are also recognized for their significant involvement in the start of germ cell death and the differentiation of gonocytes.
The proliferation of neonatal gonocytes is increased by 17β-estradiol (E2) and paracrine platelet-derived growth factor (PDGF), both of which are generated and released by Sertoli cells. PDGFR has a role in the proliferation and apoptotic regulation of newborn gonocytes. (18) E2 functions as both a pro-apoptotic and an anti-mitotic agent for newborn mouse gonocytes.
Spermatogonia derived from gonocytes are translocated to the basement of testicular cords from the lumen postnatally. Migration is essential for the correct development of spermatogonia and facilitates robust interactions between pre-spermatogonia and Sertoli cells. Following migration, apoptosis occurs in the pre-spermatogonia remaining in the core of the cord.
The ADAM-integrin-tetraspanin complex is structured inside a complex microdomain membrane referred to as the tetraspanin web. The adhesion of Sertoli cells and pre-spermatogonia is reinforced by the interaction of the tetraspanin web with F-actin. This binding, upon dissociation, results in fragmentation and structural alterations of F-actin. This portion of the Gonocytes assignment elucidates that Sertoli cells subsequently release Fas-ligand, which is locally soluble and binds to FasR in the detached pre-spermatogonium. This then results in extrinsically induced apoptosis. The gonocytes dodge apoptotic signals due to their prompt migration to the basement membrane, mostly influenced by their altered microenvironment. Therefore, it may be concluded that there exists active contact between Sertoli cells and germ cells. The quantity of supported germ cells is ensured to maintain proper physiology by triggered paracrine apoptosis.
Postnatally, gonocytes develop into type-A spermatogonia. Interestingly, in humans, the quantity of germ cells is reduced to less than half. By the conclusion of two years, all gonocytes are eliminated by the apoptosis of non-differentiated gonocytes. In humans, around the age of 3 to 4, type-A spermatogonia differentiate into type-B spermatogonia, which then develop into primary spermatocytes after migrating to the center of the testicular cord. Five
The inquiry about the Gonocytes assignment indicates that around 75 percent of germ cells in a healthy guy are transferred from spermatogonia to mature spermatozoa. Apoptotic bursts are mostly accountable for the selection of such harshness. It may be inferred from the preceding reasons that a more comprehensive knowledge of spermatogenesis and male germ cell development enhances the possibility for their use in addressing testicular infertility. Apoptosis is a crucial component of germ cell development, commencing from the early stages of embryogenesis until the conclusion of spermatogenesis. The fertility of males and their transmission of optimal genetic material to children relies on the timely and appropriately localized apoptosis of the gonads.
In the testis, it is seen that FGFs localize to many cell types, including Leydig cells, germ cells, and Sertoli cells, as indicated by Gonocyte assignment. The synthesis of DNA and cell proliferation, together with their phenotypic manifestation, have been seen to be enhanced by FGFs in cultured Sertoli cells of pigs. FGF-2 was seen to enhance Sertoli cell proliferation after 3 to 6 days of cultured cells from isolated 3-day-old neonatal or fetal rats. FGF-2 exhibited a survival factor for these cells in vitro, as it did not result in an elevation of the Sertoli cells’ [3H] thymidine labeling index. (6)
The data pertaining to the Gonocytes assignment indicate that FGF-2, as a mitogenic agent, may be essential throughout the development of the immature testis. The presence of FGF-2 in fetal Leydig cells has been confirmed by many immunohistochemical evidences. Purified cultures of pig Leydig cells were extracted from the animals to provide a main model for investigating the mechanism and impact of FGF-2 on testicular steroidogenesis. FGF-2 was seen to enhance the buildup of chorionic gonadotropin (hCG)-induced testosterone in humans after intermediate to long-term stimulation of cultured Leydig cells. Its pleiotropic function is well shown by its power to influence the maximum steroidogenic potential of Leydig cells.
The investigations conducted for this Gonocytes assignment revealed that the control of Leydig cell activity is influenced by locally generated variables. These variables also include the members of the FGF family. A study was conducted on Leydig cells obtained from rats of different ages to investigate the effects of FGF-1 and FGF-2. The study demonstrated that FGF-1 and FGF-2 increased the synthesis of basal 17?-diol and 5?-androstane-3? via immature Leydig cells, as well as the production of testosterone induced by Luteinizing Hormone in fetal Leydig cells. Seven FGF-1 and FGF-2 did not affect testosterone synthesis in adult Leydig cells; however, FGF-1 alone inhibited LH-stimulated testosterone production in mature Leydig cells. The investigation into the Gonocytes assignment indicates that the impacts of FGF-1 and FGF-2 are contingent upon Leydig cell development and differentiation.
FGF-8, extensively expressed in embryonic tissues, is crucial for the morphogenesis of the central nervous system, facial structures, limbs, and the elongation of the body axis. However, in an adult mouse, FGF-8 mRNA expression has been identified in the testis using Northern blotting. 15 Given the decline in the proliferation of mouse Sertoli cells postnatally and its cessation after puberty, one may anticipate a markedly low level of FGF-8 expression in the adult testis. This portion of the Gonocytes assignment illustrates the expression of FGF-8 and its special role in the development of the seminiferous epithelium in the testis.

Figure: Immunofluorescent detection of expression of GFRA1 and FOXO1 protein treated without and with signal inhibitors. (6)
Fibroblast growth factors (FGFs) govern several cellular functions, including cell differentiation and proliferation, throughout the embryonic development of diverse organs. Fully functioning sperm is very dependent on the testicular environment and cannot grow independently. Regulating testicular cell proliferation is essential for the preservation of spermatogenesis in the adult testis. The research discussed in the Gonocytes assignment indicates that the presence of a particular growth factor is essential for the elevated rate of germinal cell proliferation in the adult testis.
The paracrine control of testis germ cell development also includes some members of FGF. The FGF-2 supplement resulted in a substantial rise in the number of gonocytes, which were grown for six days and isolated from mice that were either freshly born or three days old. 14 The quantity of germ cells was seen to be double that of the control cultures. Research has shown that spermatogenic meiosis and the mitotic proliferation of spermatogonia are promoted by FGFs, partly via FGFRs, to improve the activity of mitogen-activated protein kinase (MAPK). (1)
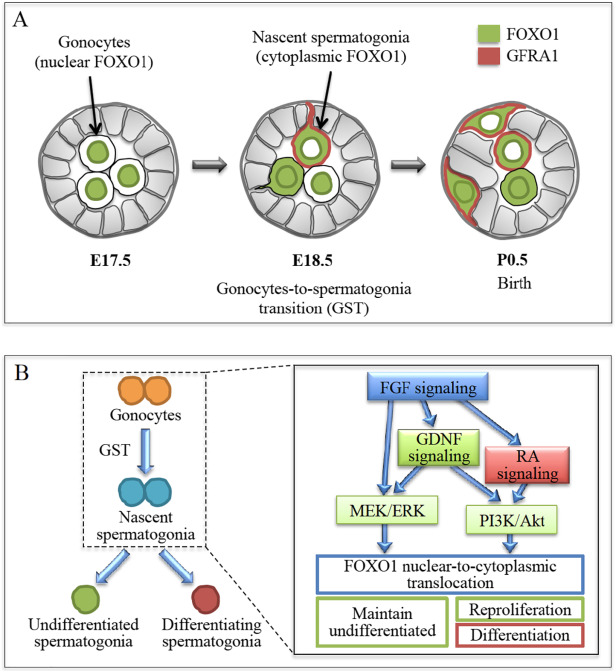
Figure: (A) GST characterization in the murine testes. GST is initiated in gonocytes. (B) GST regulation in the murine testes. FGF signaling is the basis of signaling mechanism. (4)
The phenomenon of cryptorchidism discussed above pertains to the condition when an individual's scrotum is devoid of one or both testes. This is a common congenital abnormality in the male genital tract. Approximately 1 to 4% of male neonates have undescended testis, or cryptorchidism, which may ultimately result in infertility and testicular cancer. Research indicates that infertility associated with undescended testis (UDT) arises from the formation of aberrant gonocytes, which typically transition into spermatogonial stem cells (SSC) during minipuberty (2-6 months in humans and 2-6 days in mice) or suffer apoptosis. During minipuberty, the hypothalamic-pituitary axis, regulated by FSH and indirectly influenced by androgen and LH, stimulates the transformation of gonocytes. The stoppage of gonocytes and developmental abnormalities in germ cells are the primary causes of cancer in later life for individuals with undescended testes (UDT).
The development of germ cells is a dynamic process. Following birth, during the first year, neonatal gonocytes begin to transform into adult dark spermatogonia. These adult stem cells possess a black nucleus that distinguishes them from other cells. Adult dark cells do not participate directly in spermatogenesis; rather, they ensure the provision of a requisite quantity of stem cells necessary for spermatogenesis.Adult dark spermatogonia multiply to generate light nuclei that form adult pale (AP) spermatogonia.(13) Through the process of mitosis, these cells are produced, subsequently differentiating and dividing to form primary spermatocytes, as seen in four-year-old males.
Research indicates that Gonocytes grow between the ages of 3 and 9, necessitating an optimal temperature of 33°C and enough levels of testicular hormones, including androgens and gonadotropins. Not all newborn gonocytes differentiate into AD spermatogonia; instead, they suffer involution by apoptosis. Environmental and genetic factors influence these processes.
Recently, various researchers have explored the growth and alteration of germ cells in cryptorchidism. The process of sperm cell production is termed spermatogenesis. During puberty, it starts to develop because to the elevated levels of testosterone and gonadotropins. This process involves a series of intricate steps executed sequentially, beginning with mitosis, followed by meiosis and differentiation. Autocrine, paracrine, and endocrine factors are involved at every stage. The failure to convert gonocytes into AD cells results in male infertility.
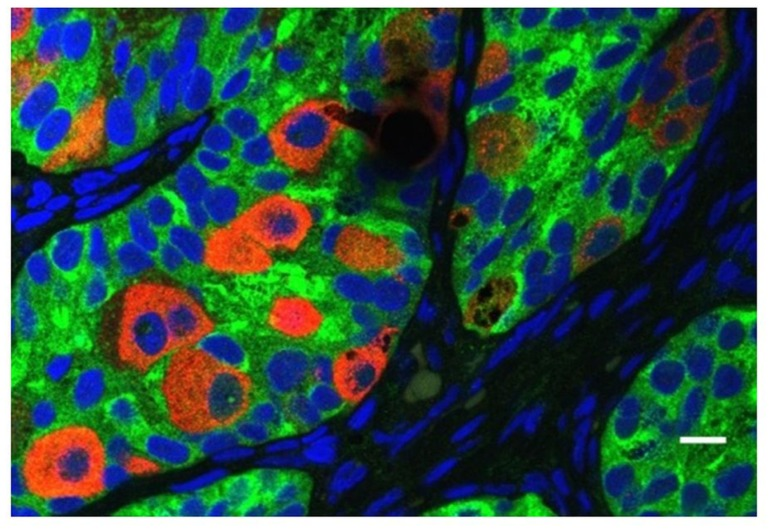
Figure: Presence of abnormal gonocytes in a boy of 6 months of age with cryptorchidism. The germ cells are indicated with mouse homolog of Drosophila Vasa and the Sertoli cells with MIS/AMH. (3)
The authors' findings indicate that the elevated temperature in cryptorchid boys is the primary cause of the improper growth of gonocytes.
Extensive research conducted on several animal models indicates that heat stress has both direct and indirect effects on germ cells, leading to disrupted transformation, maturation, and inhibition of apoptosis. This injury results from heat shock proteins and certain reactive oxygen species that impair Sertoli cells and germ cells.
Disease linked to cryptorchidism: Researchers have noted that the progenitor cells of testicular cancer resemble embryonic gonocytes. A recent study conducted for the Gonocytes assignment indicates that the elevated temperature at UD causes irregular apoptosis, allowing certain gonocytes to remain arrested and subsequently transform into cancerous cells, resulting in malignancy and infertility in adulthood due to various mutations and cellular imbalance.

Figure: Spermatogenesis and development of cancer: Malignant and normal testicular germ cell development.
The pathogenesis of germ cell cancer remains unidentified, while instabilities in the microenvironment provided by Leydig and Sertoli cells play a significant impact. The process of spermatogenesis is regulated by multiple signaling pathways originating from the Leydig cells and the surrounding environment. During the developmental phase, the expression of the insulin-like-3 gene (INSL3), which is responsible for testicular descent and gubernaculum maturation, is evident.There is a significant correlation between INSL3 and men with cryptorchidism.
Orchidopexy is a prevalent procedure in the treatment of cryptorchidism. The other two methods are inguinal surgery and laparoscopy. Preliminary surgical intervention may prevent infertility. Orchidopexy surgery is primarily conducted before the age of 2, and current research indicate that doing this surgery before the age of 1 may facilitate proper spermatogenesis by preventing gradual alterations in the testes and the loss of germ cells. This operation does not guarantee normal fertility in the future. Hadziselimovic asserts that, despite undergoing orchidopexy before to age 6, around 35% of boys were identified as infertile, regardless of the overall normal germ cell count.
A phase in infancy during which male fertility is acknowledged, occurring 30 to 90 days post-birth, is referred to as mini puberty. 10 During puberty, there is a transient elevation in certain hormones like as testosterone and gonadotropins, which facilitates the differentiation of gonocytes into A-type germ cells.This AD spermatogonia develop germ cell memory and male-specific DNA methylation mechanisms. Several investigations indicate a modest primary dysfunction in boys with cryptorchidism. It is noted in this Gonocyte assignment that shortly after birth, there is a shortage of androgen and testosterone, suggesting impaired testicular function. This hormonal insufficiency in a 3-month-old infant indicates reduced synthesis of inhibin B. (3)
Nevertheless, boys with cryptorchidism have diminished levels of testosterone and luteinizing hormone (LH), disrupted differentiation of Ad spermatogonia from gonocytes, and atrophic Leydig cells. The primary distinction between UDT and typical children is the diminished responsiveness of Leydig cells to human chorionic gonadotropin (hCG). Early administration of hCG in boys with undescended testicles may eliminate the disparity in stimulation. Five Consequently, the diminished testosterone response seen in this context of Gonocyte assignment seems to originate at the hypothalamus level, maybe attributable to inadequate activation by the Leydig cells. Multiple LH-RH assays have validated a diminished Luteinizing Hormone response to gonadotropin-releasing hormone.
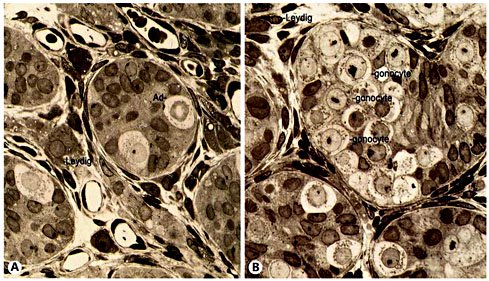
A Normal segregated germ cells in neonates throughout mini-puberty. One adult dark germ cell and two adult pale germ cells are found. The gonocyte cells are absent. B Impaired mini-puberty results in a flawed transformation of gonocytes into adult dark spermatogonia. Atrophic Leydig cells are present between the tubules, indicating poor gonadotropin stimulation.
The Human Fertilization and Embryology Authority study (2014-16) indicates that male infertility, attributed to gonocyte assignment issues, affects around 37% of the population. This study field does not get increased emphasis. This stems from the peculiar and entrenched societal ideas on the significance of femininity in infertility. The association of issues, suffering, inadequate treatment, infertility, and ultimate responsibility or blame with females is not just a female issue but also a failure of the female experience. Conversely, this problem is irrelevant for men. This intricate biological and cultural perspective of society about females results in violence and criminal incidents. Research conducted by Harvard Medical School in Boston indicates that male sperm quality diminishes with age, leading to difficulties in fertilization and perhaps impacting baby health. Although males may fail in many ways according to societal standards, women are often deemed responsible for all failures, particularly being blamed for their inability to conceive.
1.Pui HP, Saga Y. Gonocytes-to-spermatogonia transition initiates prior to birth in murine testes and it requires FGF signaling. Gonocytes assignment Mechanisms of Development. 2017; 144(B): 125-139.
2.Ignacio B, B. RA, A. PJ. Apoptosis Is a Demanding Selective Tool During the Development of Fetal Male Germ Cells. Frontiers in Cell and Developmental Biology. 2018 June; 6: 65.
3.Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction. 2015 March; 149(3): R139-R157.
4.Yang QE, Oatley JM. Chapter Nine - Spermatogonial Stem Cell Functions in Physiological and Pathological Conditions. In Rendl M, editor. Current Topics in Developmental Biology.: Academic Press; 2014. p. 235-267.
5.Hill MA. Embryology Spermatozoa Development. [Online].; 2020 [cited 2020 June 6. Available from: https://embryology.med.unsw.edu.au/embryology/index.php/Spermatozoa_Development.
6.Zhao GQ, Garbers DL. Male Germ Cell Specification and Differentiation. Gonocytes assignment Developmental Cell. 2002; 2: 537-547.
7.Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes. Proceedings of the National Academy of Sciences. 2002 September; 99(18): 11706-11711.
8.Fawzy F, Hussein A, Eid MM, Kashash AME, Salem HK. Cryptorchidism and Fertility. Clin Med Insights Reprod Health. 2015 December; 9: 39-43.
9.Docampo M J, Hadziselimovic F. Molecular Pathology of Cryptorchidism-Induced Infertility. Sexual Development. Gonocytes assignment 2015; 9: 269-278.
10.Loebenstein M, Thorup J, Cortes D, Clasen-Linde E, Hutson JM, Li R. Cryptorchidism, gonocyte development, and the risks of germ cell malignancy and infertility: A systematic review. Journal of Pediatric Surgery. 2019 July.
11.Niedzielski JK, Oszukowska E, S?owikowska-Hilczer J. Undescended testis – current trends and guidelines: a review of the literature. Arch Med Sci. 2016 June; 12(3): 667-677.
12.Cortes D, Clasen-Linde E, Hutson JM, Li R, Thorup J. The Sertoli cell hormones inhibin-B and anti Müllerian hormone have different patterns of secretion in prepubertal cryptorchid boys. Journal of Pediatric Surgery. 2016 March; 51(3): 475-480.
13.Barratt CLR, Jonge CJD, Sharpe RM. ‘Man Up’: the importance and strategy for placing male reproductive health centre stage in the political and research agenda. Gonocytes assignment Human Reproduction. 2018 April; 33(14): 541-545.
14.Mohamed RM, Adam Z, Gad M, Mazher K(. Microscopic Anatomy of Sertoli and Leydig Cells During Fetal Development in Baladi Rabbit. International Journal of Animal Science and Technology. 2018 January; 2(1): 1-5.
15.Hay-Schmidt A, Finkielman O, Jensen B, Høgsbro C, Bak Holm J, Johansen K, et al. Prenatal exposure to paracetamol/acetaminophen and precursor aniline impairs masculinisation of male brain and behaviour. Reproduction. 2017 August; 154(2): 145-152.
16.Law NC, Oatley JM. Developmental underpinnings of spermatogonial stem cell establishment. Andrology. 2020 May; 00: 1-10.
17.Szarek M, Bergmann M, Konrad L, Schuppe H?, Kliesch S, Hedger MP, et al. Activin A target genes are differentially expressed between normal and neoplastic adult human testes: clues to gonocyte fate choice. Andrology. 2018 October; 7: 31-41.
18.Chan KH, Galuska SP, Kudipudi PK, Riaz MA, Loveland KL, Konrad L. Signaling by TGF-betas in tubule cultures of adult rat testis. Gonocytes assignment American journal of translational research. 2017; 9(3): 1173–1182.
